Gases
This jar contains a gas.

Note: The numerical scores indicated in the scoring rubrics were for research purposes. Higher scores indicate higher quality argumentation. We encourage you to use a scoring scheme that matches your present goals for students.
A Why doesn't all of the gas stay at the bottom of the container?
All of the gas doesn't stay at the bottom of the container because ….
| Level | Description | Student Responses |
|---|---|---|
| 2 |
Student provides a mechanism of how the gas fills the container in terms of the motion of molecules or particles OR collisions (does not have to say “particles” or “molecules”). The answer has to get at how the gases move. |
gas molecules are always moving around in random directions. the gas particles have a tendency to occupy new areas and spread out evenly and randomly throughout the container. the gases collide with each other and bounce back in different directions. the molecules aren't dense enough to stay in one place; they float around in random motions. the gas moves in all directions. |
| 1 |
Student provides an explanation in terms of the property of gases filling containers they occupy. The answer does convey movement. AND/OR Student provides partially correct molecular-level explanation. |
They assume the form of their container. They fill up the whole container. gas molecules are relatively unaffected by gravity. Because they can exert a certain pressure to spread out in the container. Kinetic theory the gas molecules are free to move around the container as they like. also, gas molecules are very excited so they are floating around trying to get out |
| 0 |
Explanation in terms of the gas float or its density or other incorrect explanation. |
it floats up of its low density, it tends to float all around instead of just all falling to the bottom. gases tend to rise and push out towards the walls of its container. This is an example of high performance for a level zero, but there’s nothing about gases filling a container.
the lid of the container is closed so the gas wants to escape. |
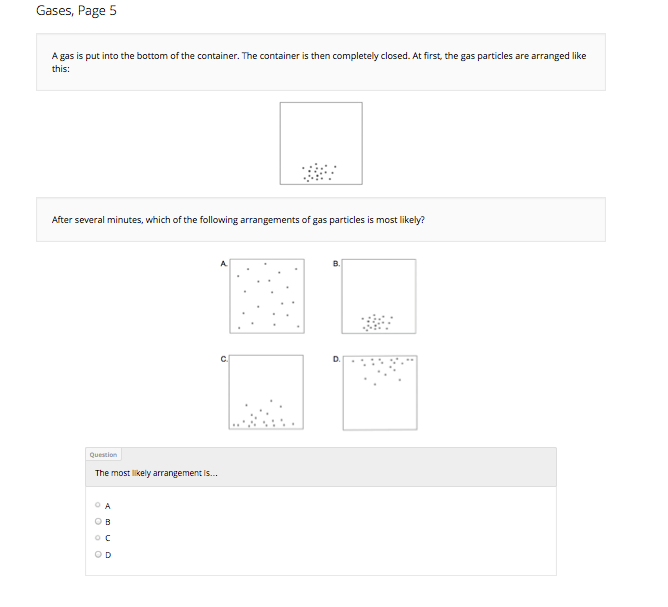
B A gas is put into the bottom of the container, then the container is completely closed. After several minutes, which of the following arrangements of gas particles is most likely?
Arrangement: …
| Level | Description | Student Responses |
|---|---|---|
| 1 |
Student selects A |
|
| 0 |
Student selects B, C, or D. |
|
C How do the particles go from where they start to where they finish, according to your selection above?
The particles ….
| Level | Description | Student Responses |
|---|---|---|
| 2 |
Student provides a complete explanation in terms of the colliding, bouncing, AND spreading out AND/OR Student talks about random movement (or moving around in all directions or all over the place). |
They start from wherever they were placed to everywhere in the container, from corner to corner. They don't have a specific path as to where they go that they follow. They just move around freely without a care in the word. bounce around in the container and spread out evenly, not in bunches. are constantly moving in a random direction. floated upwards since gases molecules move in random directions. |
| 1 |
Student provides a partially correct explanation in terms of the particles. |
The particles have no shape or volume and gas moves around freely move around and space each other out evenly spread out and occupy the volume they are given expand in the space for which they are given |
| 0 |
Student provides an incorrect, off-topic, explanation |
I am assuming there is a gas which imposes buoyancy upon the other gas. |
Sally is thinking about the gas particles and she makes the following argument:

D What reason does Sally give for her picture choice in her argument?
The reason in Sally's argument is ….
| Level | Description | Student Responses |
|---|---|---|
| 1 |
Student correctly identifies reasoning OR the student explains the argument. |
Sally gives the reason that because gas particles don't attract each other they would be spread out throughout the entire container. that "gas particles don't attract each other" Gases don't attract each other, spreading out inside the container |
| 0 |
Student does not correctly identify Sally’s reasoning. |
the ideal gas law. The gas molecules are repelling each other. that, in kinetic theory, gas molecules are neither attracted nor unattracted to each other gases do not attract, then by definition, they must repel. |
Two students are talking about gases
Their teacher tells them three facts about gases.
- Gases are made up of lots of tiny particles.
- Gases are usually less dense than liquids and solids.
- The particles move around rapidly in all directions — up, down, and sideways.
E1 Which student do you agree with more?
Student: Johnny or Sally
E2 Which fact about gases best supports the student you agree with more?
Fact: …
E1 Which student do you agree with more?
Student: Johnny or Sally
| Level | Description | Student Responses |
|---|---|---|
| 1 |
Student selects Johnny |
|
| 0 |
Student selects Sally |
|
E3 Explain how the evidence you selected supports the student's answer.
The evidence I selected best supports the student's idea because ….
| Level | Description | Student Responses |
|---|---|---|
| 2 |
Note: Scorer needs to reference whether the student selected Johnny or Sally and the evidence the student selected. Student provides complete and logical reasoning explaining how the evidence supports the claim (it explains the connection between the claim and the evidence). The student has to reference the evidence (moving rapidly) and the particles spreading out, being in all parts of the container, or that it is not just at the top of the container (the opposite). |
Note: unless otherwise noted, these examples involve students selecting Sally and the evidence of particle movement. If the particles moved around in all directions, they would not be confined to just the top of the container and would be able to move around the entire container, filling it up equally. Since the particles move around rapidly in all directions, they would be in all parts of the container. Selected student 'Johnny' and evidence 'Gases are less dense than liquids and solids.':
If it is less dense it will stay on the top of the container. |
| 1 |
Student restates the claim and/or evidence. OR Student provides partial reasoning. |
She says the gas particles are in all parts of the container and the evidence says that the particles move around rapidly in all directions. it goes well with sally's the molecules in the picture are spread out throughout the container This isn’t a 2 because it doesn’t explain how the evidence supports the claim.
|
| 0 |
Student does not provide a warrant and does not restate the claim or evidence or provides a nonsensical response. |
gas doesn't tend to attract each other Unrelated
Sally bribe me |
F1 You said you agree with Johnny more than Sally. Why do you think Johnny's idea is better?
I think Johnny's idea is better because ….
F2 Why do you agree less with Sally?
I agree less with Sally because ….
F Whose idea do you think is better? Why?
I think Johnny's or Sally's idea is better because ….
| Level | Description | Student Responses |
|---|---|---|
| 2 |
Student’s justification includes evidence (which may be a restatement) and reasoning. |
Note: unless otherwise indicated, student selected Sally. I think Sally's idea is better because since gas particles move in all directions then they are more likely to be spread out on the container than clustered on the top. gases are not bonded together like liquids and solids thereforre they can move around freely. so they would spread as widely as they can Gas particles move all around the container, meaning they are in all parts of the container. |
| 1 |
Student’s justification includes evidence or accurate reasoning. Student may refute the other argument. |
gas particles move in all directions it states a clear fact that when a gas it put into a container, it fills the entire thing. it supports the evidence Because Johnny is wrong. Unless there is something other than the discussed gas in the container
Chooses Johnny:
I agree that gases would be rising up. Chooses Johnny:
when water is steam it rises to the top of any container |
| 0 |
Student’s justification does not include evidence or reasoning. This may include restating the claim verbatim. |
Finished I think Sally is right because gas wanted to get out gas particles are in all parts of the container |
G Why do you agree less with the other student?
I agree less with the other student because …
| Level | Description | Student Responses |
|---|---|---|
| 2 |
Note: reference who the student did not select for F. Student identifies a flaw that is consistent with that is provided in the gases item and explains why it is a flaw. |
gasses move in all direction thus cannot stay in one corner Gas molecules spread out to fill their container they don't just dwell in one area. if gas particles have random, rapid, movements - up, down, and sideways, then why would it say just in place. |
| 1 |
Student identifies a flaw that is consistent with what is provided in the gases item. The student provides a counter-argument (evidence or reasoning supporting the student they agree with). |
evidence number 3 contradicts Johnny's idea scientifically, that's not how gas particles move in space gases dont just stay in place the gas spreads out throughout the container He is unreasonable |
| 0 |
Student does not identify a flaw. |
Student disagrees with Johnny:
he makes it sound as if it were a liquid or solid Not logically correct
he believes that a gas particle will stay in one part of the container. Restates the claim
|