Mixing Sugar and Water
Two students pour sugar grains into a glass of hot water. They make three observations.
-
Once the sugar is poured into the water, it is stirred. After stirring, the sugar can no longer be seen.
- Also after stirring, each student tastes the water. They both agree that the water tastes sweet.
- The weight of the water + glass + sugar is the same as the weight of the glass containing the mixture after the sugar was stirred in.
Note: The numerical scores indicated in the scoring rubrics were for research purposes. Higher scores indicate higher quality argumentation. We encourage you to use a scoring scheme that matches your present goals for students.
A Why can we no longer see the sugar?
We cannot see the sugar anymore because ….
| Level | Description | Student Responses |
|---|---|---|
| 3 |
Student response must include:
|
The sugar did dissolve but didn't reach maximum solvent.... I don't know... I'm sorry. The sugar is just mixed in with the water, but the water and sugar are not combined molecular wise... The sugar molecules are colligative? I also can't spell to save my life. We can't see the sugar it has dissolved in the water. This only occurred because the water has a high temperature, which facilitated the sugar to dissolve. However, the sugar remains itself in molecular form. If we were to evaporate the water, we can retrieve the sugar. |
| 2 |
Beyond saying “dissolved,” and there is something accurate about molecules or the sugar still being there as sugar, BUT they haven’t reached a 3. Student does not have to say “dissolved” if they accurately describe dissolving. There may be some content inaccuracy. |
Sugar molecules are bonded with water molecules and spread out in the containers. because the water molecules broke up the sugar crystals to a point when they are no longer visible to the naked eye The sugar has dissolved into the water but even though we can no longer see the sugar, it is still in the water. Only a 2 because the student demonstrates that he or she understands the sugar is still there, but there was no reference to molecules
because the sugar molecules have been scattered and have been disconnected from one another. The crystals that formed the sugar, when combined with the hot water, have dissolved. The heat from the water caused the crystals to break up into pieces so small that they "disappear." We can't see the sugar anymore because the water molecules have binded to the sugar molecules. |
| 1 |
Student states that the sugar has dissolved or mixed, but does not elaborate on what it means. There may be some content inaccuracy. |
it has dissolved into the water. Sugar is aqueous in water, therefore in dissolves in water |
Pictures of the sugar and water particles
This is a picture of the sugar and water particles before they were mixed together:
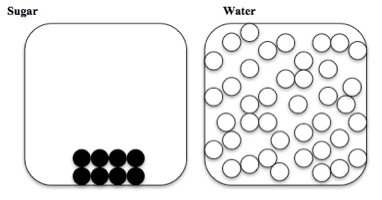
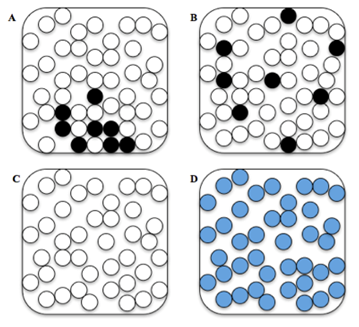
Which of these pictures best represents the water and sugar particles after they have been thoroughly mixed together?
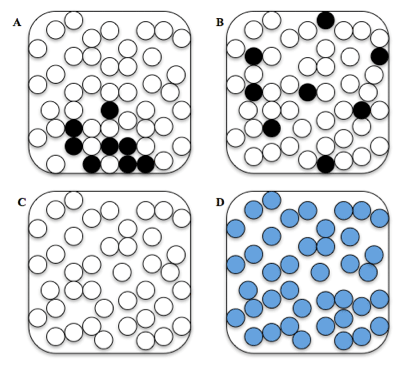
B Which of these arrangements best represents the water and sugar particles after they have been thoroughly mixed together?
Arrangement: A, B, C, or D
| Level | Description | Student Responses |
|---|---|---|
| 1 |
Student selects B. |
|
| 0 |
Student selects A, C, or D. |
|
C Why do you think the picture you chose is best?
…
| Level | Description | Student Responses |
|---|---|---|
| 3 |
Student answers B and explains that the sugar and water molecules are “evenly” or “randomly” distributed or mixed. Student gets at how they substances are mixed together. Completely coherent |
the sugar particles are spread out in the water. They are also at random which suggests the sugar has been dissolved. It shows that the sugar didn't just disappear and turn into water. Sugar and water are both present, but are now mixed. The molecules are in a random order and move around as a liquid the sugar particles are spread out in the water. They are also at random which suggests the sugar has been dissolved. |
| 2 |
Student answers B and explains that the sugar and water are mixed, but does not say how (e.g., evenly, randomly). Partially coherent OR Student explains the problems with the other representations (implying process of elimination) |
it supports my claim that the bonds of sugar molecules are broken apart and are attached to the water molecules. the molecules have broken down into atoms and dispersed in the water. The mass of the sugar is consereved but is just dissolved in the water and therefore spreads out in the liquid The sugar molecules cannot go away but they can disperse in the water. They will still be sugar molecules just not attached to any other molecules of sugar. |
| 1 |
Student answers B but provides scientifically incorrect or irrelevant explanation. |
it demonstrates that the sugar hasn't disappeared but it is still in the liquid. it's reasonable. there are more white molecules |
| 0 |
Does not answer B and provides scientifically inaccurate explanation. |
Student selected C:
It shows no signs of the sugar being visible. If I chose A or B, then that means that the sugar is visible which in water, is not. But if I chose D, then that would mean that the water and the sugar particles mixed which does not happen. Student selected D:
the water and the sugar particles will be mix together and form a new substance. Student selected D:
Because the sugar has dissolved in the water, it's not clumped at the bottom of the cup nor has it turned into water or is just floated around the cup. Student selected A:
sugar doesn't separate into separate molecules in water, nor does it completely change into a new chemical. The most reasonable answer to me is this. |
Two students discuss what they think happened to the sugar.
Laura and Mary made two additional observations of the sugar and water.
After stirring, each student tasted the water. They both agree that the water tasted sweet.
D1 Whom does this evidence support?
Laura, Mary, both, or neither
The weight of the sugar, water, and glass before it was added to the water is the same as the weight of the mixture and the glass after it was stirred in.

D2 Whom does this evidence support?
Laura, Mary, both, or neither
Laura and Mary’s teacher also tells them some information.
Matter cannot be created or destroyed.
D3 Whom does this evidence support?
Laura, Mary, both, or neither
Sometimes a substance breaks into very small pieces when mixed with another substance.
D4 Whom does this evidence support?
Laura, Mary, both, or neither
Note: Parts D2, D3, and D4 were not scored.
D1 and E Whom does this evidence support?
Laura, Mary, both, or neither
| Level | Description | Student Responses |
|---|---|---|
| 2 |
Student selects Mary and provides an explicit piece of reasoning explaining how the evidence supports the claim. |
The sweetness is due to the sugar, which means the sugar is still present If the sugar disappeared, you would not be able to taste it in the water. Plain water doesn’t taste sweet, so if the water tastes sweet than the sugar must be there. |
| 1 |
Student selects Mary, but does not explain how the evidence supports the claim. Student says they meant to choose “Mary” |
I actually chose that the statement supported Mary’s answer. She claimed the sugar was still there whereas Laura didn’t. |
| 0 |
Student does not select Mary and the reasoning is flawed. |
Both - This supports Mary because the sugar is still there because the water tastes sweeter and this supports Laura as well because the sugar could have dissolved completely and just left a sweet taste. |
At the end of the class, Mary makes the following argument:
F What is the evidence in Mary's argument?
The evidence in Mary's argument is ….
| Level | Description | Student Responses |
|---|---|---|
| 1 |
Student identifies the evidence in Mary’s argument (the total mass stays the same) or says “conservation of mass.” Additional reasoning, the claim, and additional evidence are acceptable (the sugar tastes sweet, matter cannot be created or destroyed, or a substance breaks into smaller pieces) |
The total mass stays the same. that the mass hasn't changed. Conservation of mass the sugar is there because the mass of the water plus the sugar did not change when they were mixed. The student includes the evidence in the answer and the student also includes reasoning and the claim.
|
| 0 |
Student does not identify the evidence in Mary’s argument. |
conservation of energy that if the sugar disappeared, the mass would have changed. the conservation of matter. the sugar is just changing its form, not disappear The law of conservation of matter which states that the total mass stays the same no matter what |
G Which piece of evidence best supports your answer? Explain how this evidence supports your answer.
…
| Level | Description | Student Responses |
|---|---|---|
| 2 |
Note: Reference the student’s answer to Part B since the validity of the reasoning depends on whether it is consistent with the representation the student selected. Student provides a piece of reasoning that is valid for the choice they made in Part B, explaining how the evidence supports the claim. |
Student chooses B:
Evidence: After stirring, each student tastes the water. They both agree that the water tastes sweet. Reasoning: the sugar must be assorted amongst the water particles. Student chooses A:
Evidence: Sometimes a substance breaks into very small pieces when mixed with another substance. Reasoning: parts of the sugar molecules are broken apart in the picture as it is depicted. Although this may not seem valid because the sugar is still clumped together in representation A, the student says “parts” and representation A does show some separation of the sugar particles.
|
| 1 |
Student restates the claim and/or evidence. |
Student chooses B:
Evidence: Matter cannot be created or destroyed Reasoning: sugar can't be destroyed when it was mixed with water. Restatement
Student chooses B:
Evidence: Matter cannot be created or destroyed Reasoning: Matter stays the same it just changes form or arrangement. |
| 0 |
The reasoning is not valid OR Student does not provide an explicit piece of reasoning and does not restate the claim or evidence. OR Nonsense answer |
it makes sense Student chooses B:
Evidence: The weight of the water glass and the sugar before it was added to the water is the same as the weight of the water glass after the sugar was stirred in. Reasoning: Sugar is stirred into the water so it mixes the sugar molecules up. The reasoning is not connected to the evidence.
|
You will be asked to explain why one of these pictures is not as the one you chose.
Evidence:
- After stirring, each student tastes the water. They both agree that the water tastes sweet.
- The weight of the water, glass, and the sugar is the same as the weight of the glass and the mixture after the sugar was stirred into the water.
- Matter cannot be created or destroyed.
- Sometimes a substance breaks into very small pieces when mixed with another substance.
H Pick one of the pictures above. Why do you think it is not as good? Use the evidence above, your own knowledge of mixing sugar and water, or both.
Select A, B, C, or D is not as good because ….
| Level | Description | Student Responses |
|---|---|---|
| 2 |
Note: Reference to student’s answer in Part B since the validity of the piece of reasoning depends on whether it is consistent with the student’s model. Student identifies a flaw (which may be implied to some degree) and provides a logical explanation for why it is a flaw. The critique may be scientifically inaccurate, but it must be logically consistent. |
Student selects C:
The image suggests that the sugar molecules have completely disappeared. This goes against the given evidence that matter is neither created nor destroyed. This image suggests that the sugar has disappeared and therefore destroyed. Thus it cannot be correct. Also evidence states that substances may break into smaller pieces when mixed with another substance, the image does not indicate the presence of the sugar which again would suggest the sugar to have been destroyed which, as stated, cannot be correct. Student selects D:
it is blue. No dyes were added, the sugar and water particles are white and black. Student selects A:
It hasn't fully dispersed. They would have to mix some more, or ask themselves did anyone put something random in there. Student selects A:
The sugar was stirred, not all dumped in a bag in one side of the glass. |
| 1 |
Student identifies a flaw, which may be implied to some degree. OR The student gives an explanation (evidence provided earlier in the item) without saying the flaw. |
Student selects A:
molecules don't just stay in one place they move around. Student selects D:
the water AND sugar disappeared and a blue substance magically appeared out of nowhere Student selects A:
It hasn't fully dispersed. They would have to mix some more, or ask themselves did anyone put something random in there. The explanation is unclear.
Student selects C:
Matter (sugar) cannot be created nor destroyed. The student doesn’t identify a flaw, but does provide evidence from earlier in the item.
|
| 0 |
Student does not identify a flaw or identifies a flaw that is inconsistent with the representation chosen. |
Student selects B:
It's reasonable. |
Now that you have seen some evidence and other students’ ideas, let’s return to the original question.
Two students pour sugar grains into a glass of hot water. Once the sugar is poured into the water, it is stirred. After stirring, the sugar can no longer be seen.

I Why can we no longer see the sugar?
We can't see the sugar any more because ….
| Level | Description | Student Responses |
|---|---|---|
| 3 |
Student response must include:
|
The sugar did dissolve but didn't reach maximum solvent.... I don't know... I'm sorry. The sugar is just mixed in with the water, but the water and sugar are not combined molecular wise... The sugar molecules are colligative? I also can't spell to save my life. We can't see the sugar it has dissolved in the water. This only occurred because the water has a high temperature, which facilitated the sugar to dissolve. However, the sugar remains itself in molecular form. If we were to evaporate the water, we can retrieve the sugar. |
| 2 |
Beyond saying “dissolved,” and there is something accurate about molecules or the sugar still being there as sugar, but they haven’t reached a 3. Student does not have to say “dissolved” if they accurately describe dissolving. The student’s response may have some content inaccuracy. |
Sugar molecules are bonded with water molecules and spread out in the containers. because the water molecules broke up the sugar crystals to a point when they are no longer visible to the naked eye The sugar has dissolved into the water but even though we can no longer see the sugar, it is still in the water. Only a 2 because the student demonstrates understanding that the sugar is still there, but there was no reference to molecules.
because the sugar molecules have been scattered and have been disconnected from one another. The crystals that formed the sugar, when combined with the hot water, have dissolved. The heat from the water caused the crystals to break up into pieces so small that they "disappear." We can't see the sugar anymore because the water molecules have binded to the sugar molecules. |
| 1 |
Student states that the sugar has dissolved or mixed, but does not elaborate on what it means. The student’s response may have some content inaccuracy. |
it has dissolved into the water. Sugar is aqueous in water, therefore in dissolves in water. |
| 0 |
Student provides overwhelmingly scientifically inaccurate explanation, irrelevant information, or off-topic response. |
the sugar dissolved into the sugar. The sugar molecules are so small, they are being coated by the water molecules. so all we can see is just water. it's melting. When it makes contact with the hot water, it looses it's solid structure. Molecules separate. we cant see the sugar anymore |